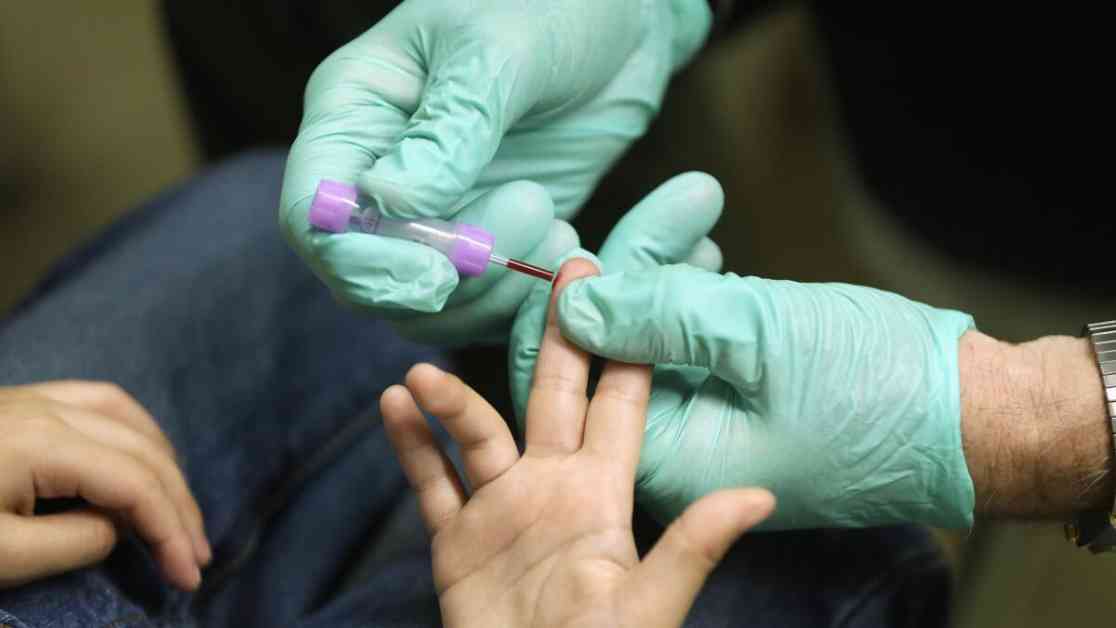
Heavy metals like lead, arsenic, and strontium can pose serious health risks when they enter the body. These toxic elements are often mistaken for essential nutrients by the body, leading to their incorporation into vital organs like the skeleton, liver, and brain. Removing these toxic metals from the body without affecting essential nutrients is a challenging task that has limited treatment options. However, a Berkeley startup called HOPO Therapeutics is aiming to change that narrative.
Recently, HOPO Therapeutics secured a significant contract worth nearly $10 million from the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services. This contract reflects the government’s recognition of the potential of HOPO’s innovative approach to treating radiation and lead poisoning.
HOPO Therapeutics has developed drugs known as chelators, which are designed to selectively bond with toxic metals in the body. By chemically transforming these reactive metals into benign molecules, the drugs enable the body to easily excrete them without affecting essential nutrients. This targeted approach could address the current limitations of existing chelators, which struggle to distinguish between toxic metals and vital nutrients.
Lead poisoning, in particular, presents a significant public health concern, especially for children. While the CDC sets a threshold for lead poisoning at 3.5 micrograms per deciliter of blood, existing treatments are often reserved for cases with higher concentrations of lead. This leaves a gap in treatment for individuals, primarily children, who are exposed to lower levels of lead.
In addition to lead poisoning, HOPO’s chelators show promise in treating radiation poisoning, which poses unique challenges due to the diverse range of radioactive elements. These elements can cause radiation sickness, cancer, and other severe health issues in exposed individuals. HOPO’s drugs target radioactive elements like uranium and plutonium, offering a potential solution for nuclear disaster scenarios.
One of the key advantages of HOPO’s drugs is their oral administration, making them more accessible and practical for emergency situations. This ease of use could be crucial in responding to nuclear incidents or other radiation exposure scenarios where swift intervention is necessary.
HOPO Therapeutics is now focused on advancing its drug development process, including scaling up production for large-scale manufacturing and conducting human trials to assess safety and efficacy. The ultimate goal is to secure FDA approval for their innovative chelators, paving the way for a new standard of care in treating radiation and lead poisoning.
By pioneering targeted and effective treatments for toxic metal exposure, HOPO Therapeutics is poised to make a significant impact on public health and emergency response efforts. The government’s support and funding underscore the importance of advancing innovative solutions to address critical health threats, ensuring a safer and healthier future for all.